
Opening hours ( 4 Nov - 10 Nov)
-
This week| Next week
-
Monday
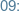
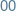 -
- 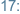
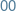
-
Tuesday
 -
- 

-
Wednesday
 -
- 
-
Thursday
 -
- 

-
Friday
 -
- 

-
Saturday
closed -
Sunday
closed
Opening hours (11 Nov - 17 Nov)
-
This week |Next week
-
Monday
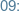
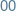 -
- 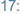
-
Tuesday
 -
- 
-
Wednesday
 -
- 
-
Thursday
 -
- 
-
Friday
 -
- 
-
Saturday
closed -
Sunday
closed
Late night shopping
Late night shopping unknown
Late night shopping
Sunday shopping unknown